Cuba opted to develop its own vaccine against COVID-19. The aim was to achieve health sovereignty in terms of immunization and to generate income exporting it to other countries. Thirteen months after the vaccine was approved, and amidst informative obscurity, the island has sold a minimum amount of doses to allied countries, and the vaccine is still not acknowledged by the WHO and the scientific community.
This publication was collectively developed by journalists who are members of the #CONNECTASHub*
In “Abdala”, a play written by Jose Marti, the Cuban intellectual of the 19th century discussed the pro-independence feeling that overwhelmed him when he was just 15 years old. In the narration, the hero of an African country is getting ready to fight in a foreign invasion. In this post-pandemic world, “Abdala” is also the name given to the first Latin American vaccine, developed by the CIGB (Genetic and Biotechnological Engineering Center), one of three vaccine manufacturing laboratories in Cuba.
The name Abdala, as well as the names of the other four vaccine prototypes being developed in the island -Mambisa (the name of guerrilla groups that fought for the Cuban independence in the 19th century), Soberana 01, Soberana 02 and Soberana Plus-, reflect the aspirations with which the Cuban government decided to face the ‘foreign threat’ of the pandemic: developing their own vaccine instead of buying them from other countries. In theory, this plan guaranteed the autonomy of the immunization process and, incidentally, sales to other countries would bring in foreign currency.
But, even though Abdala was authorized for emergency use on July 9th, 2021 by the CECMEC (Center for State Control of Medication, Equipment and Medical Devices) and that as of August 27th, 2022, 90.1% of the Cuban population had been vaccinated with it, the promise of exporting the vaccine has not materialized. The same happens with the other four vaccines developed by the IFV (Instituto Finlay de Vacunas) and the CIGB.
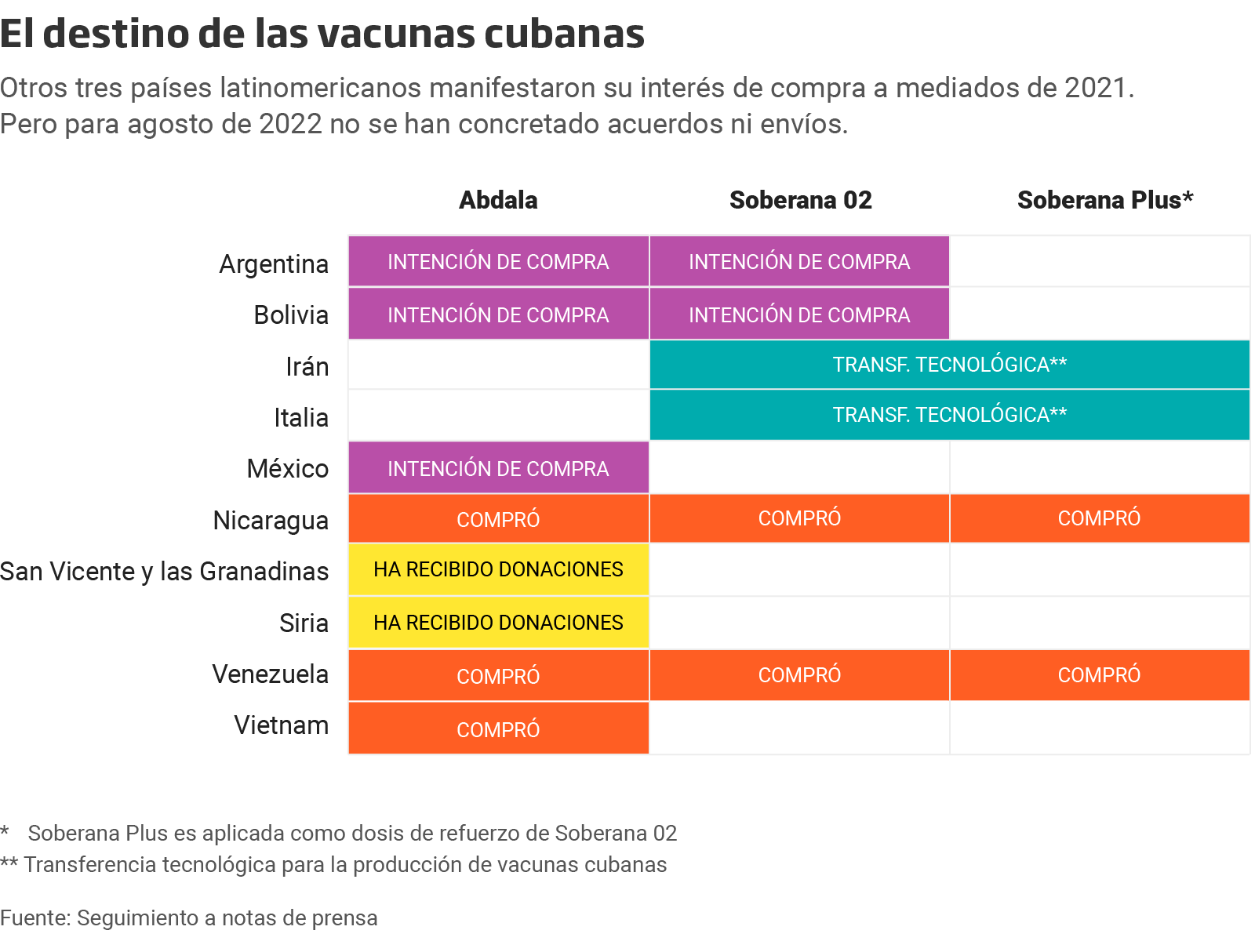
Soberana 02 and Soberana Plus are also authorized for emergency use by the CECMED since August 20th, 2021. But a year after these approvals and according to the scant information emerging from the island, Cuba has only sold doses of its vaccines to Venezuela, Nicaragua and Vietnam, countries that are inside its reduced circle of geopolitical relationships. Additionally, it has sent symbolic amounts to Syria and Saint Vincent and the Grenadines. For Latin America, public information available shows that the total amount of doses agreed has not been exported yet.
The international scientific community is still hesitant about the effectiveness of Cuban vaccines. Alejandro Risquez, epidemiologist and Director of VacuVen, Venezuela’s vaccination center, explains that mistrust in the vaccines can be explained by the fact that important stages are still pending. He explains that these types of biologics must earn trust in two scenarios: internationally, with the WHO’s approval; and in each country, which decides whether to buy it or not. One is not necessary to get the other, Risquez clarifies. "For example, Sputnik -the Russian vaccine- has not been approved by the WHO. However, it has been accepted by more than sixty countries. They (the manufacturers) present their figures, and the perception of countries where it has been administered shows that it was fine." But in the case of Cuban vaccines, he adds, trials for stages III and IV have not been published, and therefore they have not gained the credibility of the WHO and almost any country.
On September 1st, 2021, Eduardo Martinez Diaz, President of BioCubaFarma (the group that centralizes the operation of 32 companies that produce medication and equipment for export), announced on Twitter that Cuba would begin conversations required for the authorization of Cuban vaccines by the WHO. By August 2022, and according to the WHO’s chart of the status of COVID-19 vaccines within WHO EUL/PQ evaluation process, with the latest update on July 7th, the organization is still awaiting documentation on Soberana 01, 02 and Plus to kickoff the process. Only last June, data was submitted to begin the dossier review for the Abdala vaccine.
The WHO explained to CONNECTAS that “the extension of the process of emergency use listing depends on the quality of the data presented by the vaccine manufacturer and on the data fulfilling WHO criteria”. A similar explanation was given by Martinez Diaz in January 2022, via Twitter. He asserted they were “adapting the documentation to submit to the WHO."
lang="es">Las primeras vacunas #COVID19, desarrolladas en A. Latina y el Caribe son #Abdala, #Soberana02 y #SoberanaPlus, son vacunas cubanas. Lograron el Autorizo de Uso de Emergencia otorgado por el CECMED, una Autoridad Regulatoria reconocida por la OMS y de referencia en la región. pic.twitter.com/ookFEVn1dy
— Eduardo Martínez Díaz (@EdMartBCF) December 26, 2021
A Risky Bet on the Vaccine
The pandemic struck the Cuban economy hard. Since 2017, the country is going through one of the worst economic and energy crises, which resulted in the social unrest of July 2021. An unprecedented phenomenon in Cuba’s modern history. Measures taken worldwide to control the pandemic (border closures and isolation) affected the island’s tourism, from four million visitors in 2019 they went to one million in 2020, a significant decrease for the national resources.
Amidst this difficult scenario, the island decided to bet on one of its economic pillars: the production and export of medicines, equipment and medical services. The IFV and the CECMED are Cuban entities that have been acknowledged by multilateral organizations and the international scientific community based on the island’s 40-year track record in vaccine production. The two laboratories that developed the biologics against COVID-19 have manufactured ten different types of vaccines, at least. The IFV invented the VA-MENGOC-BC, the first vaccine against meningococcus in the world.
Alongside the crisis that was made worse by the pandemic and the downfall of tourism, Cuba deployed medical missions to forty countries in the world, promoted at least three of its medications as effective against COVID-19, and the largest bet of all: developed five vaccine prototypes.
In spite of a dramatic reduction of its income, on november 1st, 2021, Cuba inaugurated the CIGB-Mariel Biotechnological Industrial Complex, with the goal of manufacturing medications and vaccines, including part of the development of drugs that the island has developed to treat and prevent COVID-1: the Abdala Vaccine and Jusvinza, Herberprot-P and Heberferon medication.
After Adbala and the Soberana vaccines were approved, Cuba set the goal to manufacture 100 million doses by 2021 with the support of allied countries. The objective was to vaccinate its entire population and export, at low cost, to countries with less resources. It also intended to export its vaccines to the European and African market.
So far, Europe has been limited to the participation of Italian volunteers in clinical trials and to a recent signature of a memorandum of understanding to begin joint production. According to a follow-up on Cuba’s official press, the proposed goal seems to have been achieved only with Iran, an ally of the Cuban government in the development of Soberana 02.
The economic difficulties faced by the island constitute a big challenge, Risquez comments: “Cubans have attained global positioning as a small country with significant progress in biotechnology. Nevertheless, it is undeniable that the objective was not fulfilled in terms of the COVID-19 vaccine. They have endured economic hardship and that must have affected logistics. They invested a lot of time and money enhancing their industry, and they did. But the industry is not in the capacity to withstand a financial issue such as this one. They have limitations to recover equipment, input and funding.”
On January 10th, 2022, the CABEI (Central American Bank for Economic Integration) granted Cuba EUR 46.7 million to manufacture 200 million vaccines against COVID-19 and to strengthen the country’s biopharmaceutical industry, as per the press release posted on the Bank’s website.
However, the objective of the loan’s resolution, also posted on the website, does not include the production of 200 million vaccines; it is limited to an allocation of resources to “strengthen the Cuban biopharmaceutical industry to fight against COVID-19 in Cuba and the region”. The resolution also sets forth that the resources will be implemented through the UNPD (United Nations Development Programme).
It has been seven months since the loan was granted, but the resources have not been executed. “The review and the signature of the agreement that supports the allocated funds has not concluded, so the funds have not been transferred and the implementation process by the UNDP has not begun,” the organization’s press team responded to CONNECTAS.
CONNECTAS sent a request for public information to the CABEI in order to understand the reasons for the delay. Additionally, BioCubaFarma, IFV and the CIGB were also contacted to get a version of the scope and the challenges of Cuban vaccines against COVID-19. Yet, as of the publication date of this feature, they had failed to answer the request.

In September 2021, Nguyen Xuam Phuc, President of Vietnam, visited the CIGB, the laboratory that developed the Abdala vaccine
Photo: Agencia Cubana de Noticias
Political Allies, Target Markets
Iran is the most important ally in the manufacturing and commercialization of Cuban vaccines. Through the Pasteur Institute and in alliance with CIGB, the Persian country is the joint creator of the Soberana 02 vaccine. It authorized its emergency use before Cuba, in April 2021, and conducted the stage III of the clinical trial in its territory. Moreover, in May, it inaugurated a manufacturing plant for the vaccine, which is commercialized in Iran as PastuCovac.
Venezuela, for its part, was the first country to buy Cuban vaccines. It even imported the first batch in June 2021, a month before the approval of the island’s health authority. Back then, both nations entered into a purchase agreement for 12 million doses of Abdala. However, a follow-up on the official reports issued by Transparencia Venezuela, the South American country has received only 7.886.400 doses of the vaccine, i.e., more than 4 million doses are pending to fulfill the first agreement. According to official statements, Venezuela has gotten 167,000 doses of Soberana 02 and 1 million doses of Soberana Plus. There is no information pertaining to new batches, and the Venezuelan government hasn’t reported figures since February 2022.
When the import of the first batch of Cuban vaccines was announced in June 2021, and then again in November, when the government said it would be administered to children, the Venezuelan scientific community (Academia Venezolana de Medicina, Sociedad Venezolana de Infectologia, Asociacion de Investigadores del Instituto Venezolano de Investigaciones Cientificas and Sociedad Venezolana de Puericultura y Pediatria) warned that the Cuban biologics did not have peer approval nor the authorization of an international organization. For these reasons, they argued, the vaccines were still in the experimental stage, and volunteers should give informed consent.

Cuban vaccines were first administered in June 2021 in Venezuela
Photo: El Pitazo / Ronald Peña
Nevertheless, according to the reports of Venezuelan press, that protocol was not fulfilled. Angello Gomez, who lives in a residential complex in Caracas’ Fuerte Militar, began his vaccination schedule with Abdala on June 26th, 2021. Although he did it voluntarily and because it was conveniently close to his home, he was never informed by the health personnel that the vaccine was not yet approved by health organizations and that it was in the experimental stage.
The document he signed, which was reviewed by the team at CONNECTAS, states the name of the biological, the global context of the COVID-19 pandemic and the possible side effects of the vaccine.
A similar situation took place in Nicaragua. The Central American country received 1,200,000 doses of Abdala and Soberana 02 vaccines in October 2021. That was the first batch of vaccines that were set to arrive between that date and December 2021; the agreement contemplated a total of seven million doses of Abdala, Soberana Plus and Soberana 02, to be administered to two million children and teenagers.
After the official announcement, the AMEN (Asociacion de Medicos Nicaragüenses en el Exilio) issued a statement expressing concern. “In the absence of adequate scientific information, vaccines offered to Nicaraguan children are in breach of bioethical standards because parents were not told that said vaccines were in the experimental stage,” La Prensa, the Nicaraguan newspaper, wrote.
Nicaraguan parents did not have any other option in 2021. In a context where nobody dares to speak up out of fear of retaliation from the Sandinista government, CONNECTAS attained the testimony of Adriana, a mother who decided to vaccinate her asthmatic daughter in December of last year. She did not complete the vaccination schedule due to family trips and mounting distrust against the Cuban vaccine. But, seven months later, her daughter’s school reported cases of infected parents and Adriana thought the time was right to get her daughter the next dose.
Late in June, a vaccination brigade stopped by her neighborhood and she was told that they didn’t carry the Cuban vaccine anymore, but that her daughter could get vaccinated with Pfizer because she was asthmatic. A month before, the country had received a batch of 650,600 doses of this pediatric vaccine as a donation from the United States. In the early days of August 2022, Adriana resumed her daughter’s vaccination schedule.
This case proves that Nicaragua’s vaccination program was completed with the Cuban vaccines, but it was impossible to confirm this information because the Sandinista government has not made it available.
To date, according to the Nicaraguan outlet Confidencial (which follows-up on official statements), only three batches of the Cuban vaccines have arrived in the country, amounting to a total of 3.1 million doses. According to an investigation by the same outlet, the Nicaraguan government paid USD 7 per Cuban vaccine. The journalistic investigation revealed that Nicaragua paid USD 2.5 more for each unit of the Cuban vaccine compared to what it paid per unit of the AstraZeneca vaccine, which it bought through the revolving fund.

After vaccinating their children with Cuban vaccines, Nicaraguan parents decided to complete their children’s schedules with other vaccines
Photo: Silais Managua
Saint Vincent and the Grenadines received 300 doses of Abdala as a symbolic gift from Cuba when Prime Minister Ralph Gonsalves visited Havana to attend the Summit of the Bolivarian Alliance for the Peoples of Our America – Peoples' Trade Treaty (ALBA-TCP).
But exports of Cuban vaccines transcend the American continent. In September 2021, after the health authority of Vietnam granted the authorization of emergency use, the Asian country agreed to buy five million doses of the Abdala vaccine from Cuba and to provide technological transfer to manufacture the doses. Additionally, Cuba donated 120,000 doses of this biological to Syria in January 2022.
Empty Words
In April 2021, Benjamin Blanco, Bolivia’s Vice Minister of Foreign Trade and Integration, and Alejandra Hidalgo, Vice Minister of Health Insurance, met with Danilo Sanchez, Cuban delegate of business, to coordinate the purchase of the Cuban vaccine. “They have started vaccinating and I hope that they achieve a significant amount of immunized people by May, this will contribute to the trials,” the website of Telesur reported the words of Blanco.
Jhonny Oscar Mamani Gutierrez, Governor of the department of Potosi, announced in June 2021 that he was interested in purchasing doses of the Abdala vaccine to immunize 10 to 18-year-olds, the doses that Bolivia had were just not enough. A commission consisting of Huascar Alarcon, the epidemiologist at Sedes (the Departmental Health Service) in Potosi, as well as Juan Tellez, Advisor to the Governor, traveled in July 2021 to meet officials and doctors of the Cuban embassy in La Paz to be briefed on the vaccine’s effectiveness and efficiency.
Alarcon explained that they ruled out purchasing the vaccine because it was not in COVAX, it was not authorized by the WHO, and it was in the clinical stage. “As a department, we had the budget. But there was a lot of controversy surrounding the vaccine, was it patented or not… We requested to purchase it through the Ministry of Health, but that did not come through.”
The Director of Bolivia’s PAI program (Programa Ampliado de Inmunizacion), David Choqueticlla, and the National Director of Epidemiology of the Ministry of Health, Freddy Armijo, confirmed to CONNECTAS that there is no ongoing negotiation to acquire the vaccine.
The experience is similar in Mexico. In December 2021, COFEPRIS (Comision Federal para la Proteccion contra Riesgos Sanitarios), which is recognized by the Pan American Health Organization, authorized the emergency use of the Abdala vaccines in the Mexican population because it deemed it safe and effective.

Five months later, Mexican President, Manuel Lopez Obrador, guaranteed that the Cuban vaccine would be administered to children under 11. “We are going to purchase a vaccine they are manufacturing for very small children, which has had great results. This vaccine is for children, for kids, basically for COVID-19 in the first stage for children over 2,” El Pais reported.
At that moment, Mexican scientists made a statement against the announcement. They argued that there was not enough scientific evidence to confirm the safety and efficacy of the vaccine in children. Moreover, COFEPRIS, which also regulates the sales of medication in the country, has not authorized the use of the vaccine in that specific population.
The Senate also questioned the decision announced by Lopez Obrador, the Secretary of Health was requested in a letter signed by representative Ana Lilia Herrera Anzaldo to communicate and share the evidence pertaining to the safety and efficacy of Abdala administered to children and teenagers.
lang="es">Se está analizando el expediente técnico de la vacuna cubana #Abdala. El análisis está a cargo de @COFEPRIS y @Conacyt_MX, no hay duda sobre su calidad y seguridad. Se está evaluando la evidencia para su uso en población pediátrica @HLGatell pic.twitter.com/PKFbTtbg7R
— Instituto de Salud para el Bienestar (@INSABI_mx) June 7, 2022
Giorgio Franyuti, General Director of the Medical Impact NGO, explains that COFERPIS has the powers to issue authorizations in multiple ways, i.e., emergency use. “But pediatric authorizations require demonstrating a health emergency for that population. Out of 1,080 minors who died of COVID-19 in Mexico, at least 350 were among the pediatric population set to get the vaccine, and this figure is not relevant enough in terms of public health for the emergency authorization. That is why they didn’t get it.”
He also states that to fulfill the promise of the Mexican government, the trial of the III stage in children and teeneagers must be done again: “Unfortunately, this vaccine does not have a lot of international collaboration to replicate its trials. Additionally, other options achieve better immunization. (Although) I can not guarantee nor verify this, because the information has not been provided to the scientific community for suitable comparison. Seeing as we cannot purchase it, we definitely shouldn’t be administering it.” The reality is that Abdala is not yet available and is not administered in Mexico.
Likewise, the purchase and support of the Cuban vaccine in Argentina has been nothing but good intentions. In May 2021, the country’s Minister of Health, Carla Vizzotti, met in Havana with Cuban President, Miguel Diaz Canel. Argentina “is working hard to strengthen ties, access the vaccine and collaborate in everything it can, including the supply of consumables, the option to purchase and the support to escalate manufacturing and move on with our regulatory entity -and Cuba’s- in the study of the internal analyses of stage III to extend the likelihood of research in places such as our country, which could help position for the vaccine in a significant way,” as reported in a news clip by the official website of the Presidency of Argentina.
But that was it. To date, there is no public information in terms of progress made in the lines that were suggested.

In May 2021, the Minister of Health of Argentina, Carla Vizzotti, met with Cuban President Miguel Diaz-Canel and declared the support and intention to purchase Cuban vaccines
Photo: website of the Presidency of Argentina
Cuba Persists
Even though Cuba has failed to materialize the export of its vaccines to allied countries in Latin America, in 2022, it began the requirements to expand to markets in Europe and Africa.
In March, the directors of the IFV and BioCubaFarma traveled to Europe with the goal of promoting the results of the vaccines. At the time, the Director of the IFV, Vicente Vere, said that the objective of the visit was to find a formula to sell the Cuban vaccines in Europe; and even in Africa, with the help of Italy. “We have tried to bring awareness to Italian society, we have an important instrument to safely vaccinate children. And we are reaching out to different actors because there are barriers for products developed and manufactured in Cuba to enter the European market," Diario de Cuba reported.
That same month, 30 Italian volunteers went to Cuba to participate in the clinical trial of the Soberana Plus vaccine. The purpose was to verify if this biological worked as booster dose in people who had gotten other vaccines against COVID-19.
In April, the IFV, the italian company Adienne Pharma & Biotech and the AICEC (the Italian Agency for Economic and Cultural Exchange with Cuba) signed a memorandum of understanding to begin production of the Cuban biological. It is unknown if this process has begun.
But the reach of Cuban vaccines in Europe is not limited to Italy. On July 28th, the Center for Examinations and Tests in Health Service of the Ministry of Health of Belarus authorized the use of Soberana Plus in that country. However, as of August 2022, there is no information to indicate if doses are being exported to other European countries.
The Cuban pharmaceutical industry’s recognition and track record has not been enough to leverage its intention to become a big supplier of COVID-19 vaccines. “The hepatitis vaccine was an important accomplishment for Cuba. Costs went down worldwide and the fact that it sold throughout Latin America turned the island into a powerful manufacturer. But they did not deliver with the COVID-19 vaccine. They could not fulfill the expectations,” Risquez concludes.
* Reporting
Luis Fernando Cantoral (Bolivia)
Carlos Gutierrez (Mexico)
Claudia Smolansky (Mexico)
Grisha Vera (Venezuela)
Collaboration
Team of reporters at Confidencial (Nicaragua)
Writing and Edition
Grisha Vera (Venezuela) - Leonardo Oliva (Argentina)